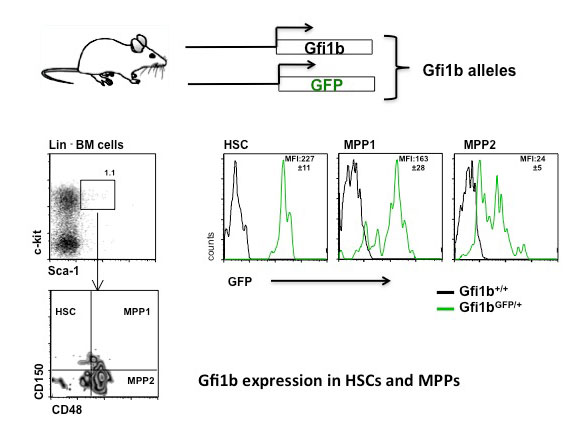
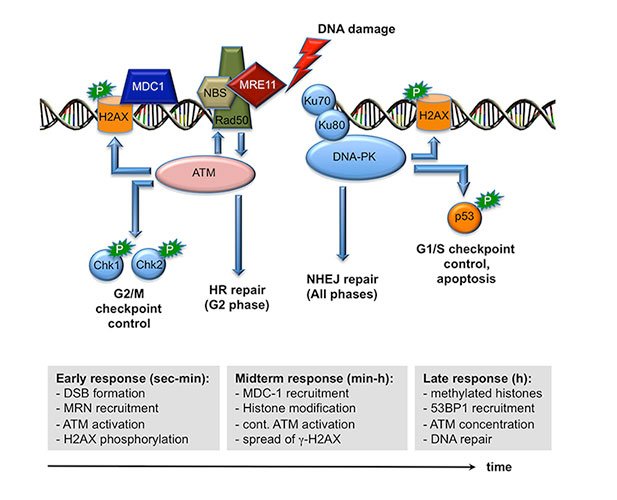
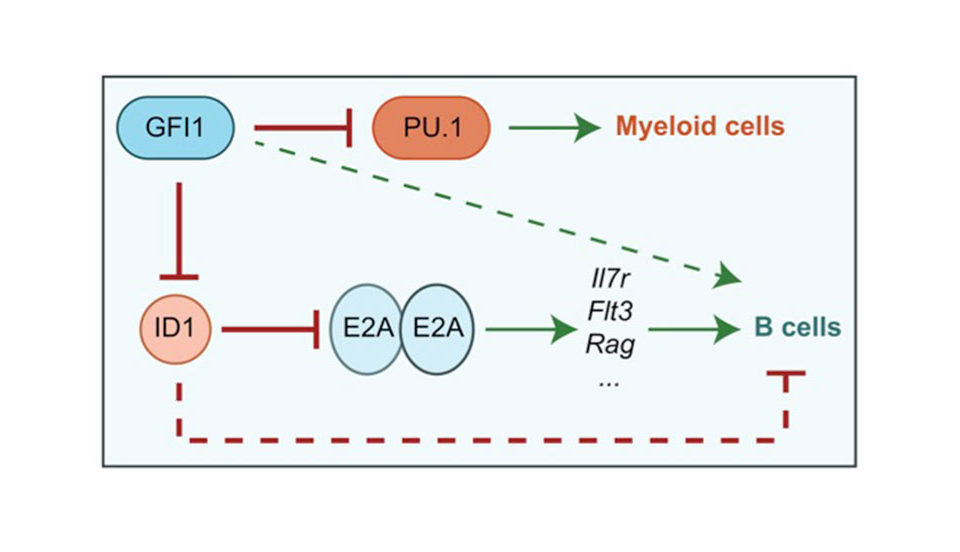
In leukemia, a form of blood cancer, cells from the bone marrow multiply without control and invade the bloodstream and other organs. This compromises the function of the red blood cells (oxygen transport) and the white blood cells (immune defense) and leads to severe illness and death. Patients with leukemia cannot always be cured by chemotherapy and have to undergo stem cell therapy. During this therapy, the entire blood producing system (and with it all cancer cells) of a leukemia patient are killed off by irradiation and is then replaced by a new and healthy blood producing system by giving patients blood stem cells from a healthy donor by infusion. These blood stem cells are then capable of regenerating the entire blood producing system. However this therapy still fails in about 20% of all cases and the leukemia patients die during the procedure mostly because the transplanted blood stem cells do not give rise to enough new blood cells in the patient. One reason is that blood stem cells are normally very passive and are dormant in a defined place in the bones (called the endosteal niche). After transfusion, the transplanted done stem cells go again back to their niche this time in the patient, where they are kept in a dormant state. We have observed that a protein called Gfi1b regulates where the stem cells reside in the bone and whether they are active or not. We have generated a mouse model enabling us to switch the Gfi1b gene off, and when we do this, suddenly the stem cells become activated, start to expand drastically and also leave their niche but do not loose their function. We wish to study, how this regulatory protein (Gfi1b) works and in particular, how it can determine where blood stem cells are localized and how it restricts their activation. The ability to activate and mobilize stem cells by antagonizing Gfi1 would certainly be beneficial for leukemia patients that undergo a stem cell therapy and have received a stem cell transplant.